What are asphaltens?
The present trend in the petroleum industry is an increasing demand for
light products. In order to meet the market demand, refineries
 convert a portion of their residuals into light fractions. This conversion
results in production of modern heavu fuels which contains a greater
concentration of sulphur, vanadium and asphaltens.
convert a portion of their residuals into light fractions. This conversion
results in production of modern heavu fuels which contains a greater
concentration of sulphur, vanadium and asphaltens.
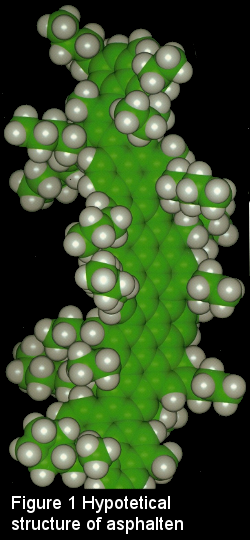
Asphaltens are considered as part of the "bottom of the barrel". They constiture the non-volatile, high molecular weight fraction of petrloeum. In addition, they are defined as a solubility class since according to standard methods, they are nonsoluble in hepane (ASTM D3279 or IP 143). Consequently, they remain in solid form in the fuel as well. Although their exact molecular structure is difficult to define, it is hypothesized that asphaltens consist of condensed polynuclear aromatic rings bearing alkyl side-chains. (See Figure 1)
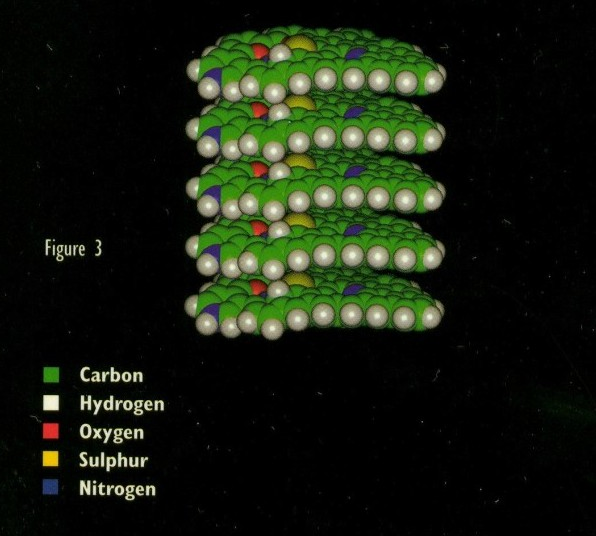
The condensed aromatic rings exist in the form of a nonhomogeneous flat sheet (See Figure 2). In the fuel the asphaltene sheets remain despersed. Howewer, whitout the presence of a fluid thay have tendency to be attracted towards each other thus resulting in the formation of an agglomeration. The structure of the agglomeration is similar to that of a book: a compact stack of thin sheets (See Figure 3).

Why asphaltens a problem in Boilers?
Asphaltens exists in the fuel in a dispersed state, held in this condition by the resins. Thay hace three (3) characteristics that make them problematic to combustion system:
- they constitute the largest aromatic fraction in petroleum as well as being the highest molecular weight component
- they have no definite melting point and therfore remain in solid form thus contributing to the carbon residue content of the flue gas
- they agglomerate to form a book-like structure
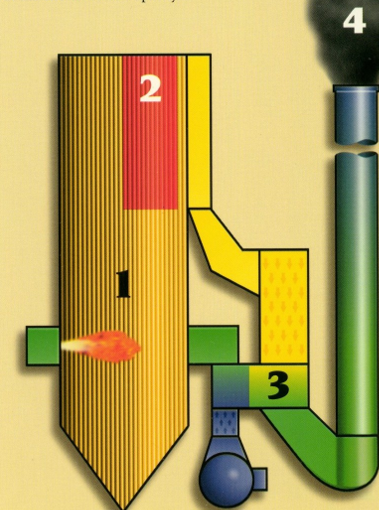
Liquid fuels does not burn in a liquid state, but rather in a gaseous state. In order to burn a liquid fuel, it is first necessary to atomize the fuel to form a mist of vvery small droplets. As these droplets move towards the flame front, the temperature rises rapidly resulting in the vaporization of the light fraction of the droplets. The solid asphaltens, which make up the heavy fraction, agglomerate into compact, book-like structures as combustion of the volatiles takes place (See Figures 3 and 4).
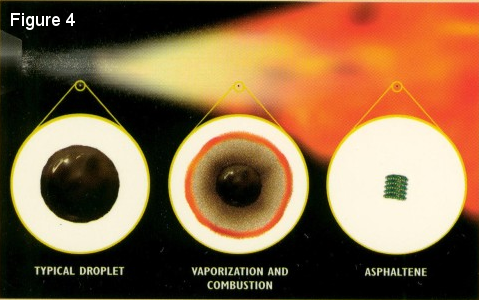
These large look-like solid hydrocarbons are very difficult to
burn since their combustion must take place in the solid phase.
The mechanism by which solid phace combustion takes place
is oxygen diffusion through the pores of the solid. Diffusion whthin
a solid is fenerallt a slow process and it therfore limits
the speed at which cobustion takes place. In addition, since
the burnout time of a solid is proportional to the square of its
equivalent diameter, the agglomeration of the solid asphaltens
adds to the difficulty of their combustion since the burnout time
is greatly increased. A typical residence time of 0.1 second in the
flame zone is insufficient to complete combustion.
Unburned carbon residues are formed, which contribute anywhere
from 40% to 80% of the total particulate emissions. Resulting problems
in the example of boiler are as follows:
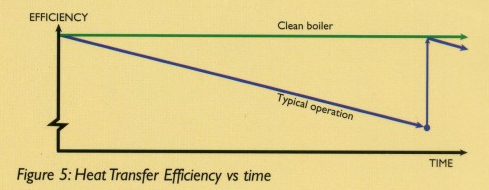
Some of these residues will deposit inside the boiler, forming an insulating layer on the tubes thus reducing the heat transfer efficiency. The result will be a less efficient operation (See Figure 5) since the outlet flut gas temperature will progresively increase over time.
2. High temperature corrosion
Unburned carbon residues also act as a carrier for non-combustibles such as vanadium. Carried over by the flue gases, some of these residues will deposit on superheater tubes. The environment in the superheater is very oxidizing: due to high temperatures and the presence of residual oxygen.
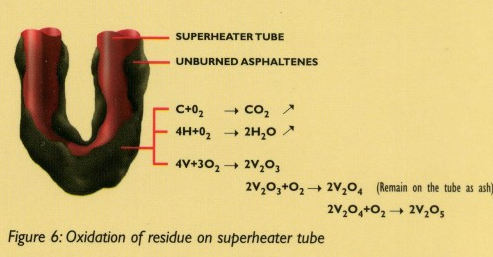
In this environment, the hydrocarbon content of the deposited residues will slowly oxidize to gaseous CO2 i H2O, which will be evacuated with the flue gases. However, the vanadium will also be oxidized to V2O2 (vanadium trixide) which is a solid ash with a melting temperature of 1970°C.
Slowly, the vanadium trioxide (V2O3) will further oxidize to vanadium tetraocide (V2O4) with a melting temperature of 1967°C and then to vanadium pentoxide (V2O5) with a melting temperature ranging from 550°C to 700°C in the presence of sodium oxides (See Figure 6).If the flue gas temperature exceeds the melting temperature of this ash, the deposit becomes molten and coats the superheater tubes. It is this molten form of the deposited ash which is promarily responsible for high temperature corrosion inside boilers.
3. Low temperature corrosion
In addition to limiting heat transfer efficiency and causung frequent maintenance, unburned carbon residues that deposit in air preheaters will act as a carbon media-filter by absorbing sulphur oxides from the flue gas. When the air preheater wall temperature falls below the dew point, water vapour (H2O) condenses on the wall surfaces and reacts with the SO3 present in the residue. Sulphuric acid (H2SO4) is formed, resulting in cold end corrosion.
4. Stack opacityFinally, these unburned carbon residues are carried through the stack creating a visible black plume. It was found that due to their hight light absorption coefficient, unburned carbon particles can contribute up to 75% of the effective stack opacity.